Dr. Xiangbing Qi's laboratory first found the chemical selective migration and cross-coupling chemistry of alkyl zirconium
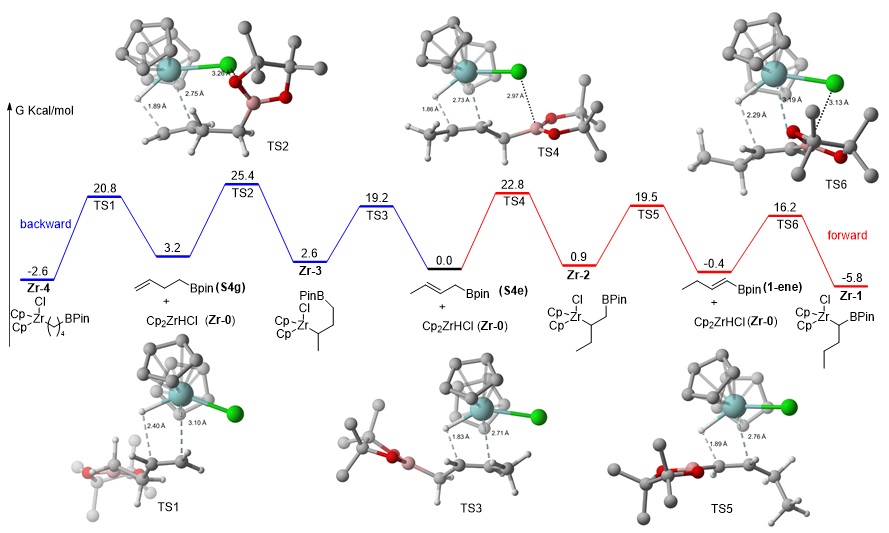
Due to the high capacity of rapid constructing complex molecules, especially three-dimensional molecules, the chemically selective cross-coupling reaction of bimetallic alkyl reagents has a wide range of synthetic potentials. Among known bimetallic reagents, gem-borazirconocene alkanes are recognized as particularly valuable synthons that possess great synthetic application due to its different reactivity of carbon-zirconium bond, convenient operation, and good functional group tolerance. The polarity of carbon-zirconium bond and carbon-boron bond in alkyl zirconium boron reagents is quite different, which allows researchers to selectively functionalize the reagents through various reactions such as nucleophilic substitution or nucleophilic addition. Compared with the extensive research of this reagent in halogenation, deuteration, amination, Michael addition and nucleophilic addition reaction, the wide application of this reagent in cross-coupling reaction has not been reported. While cross-coupling reaction of primary alkylzirconocene reagents has been reported recently by our group, to date, no general cross-coupling reaction has been developed for secondary alkylzirconocene including gem-borazirconocene alkanes, likely owing to the lack of available π-systems to stabilize the binding capability of zirconium to achieve suitable transmetalation. Furthermore, the considerable steric hindrance caused by the zirconium complex decreases the nucleophilicity of the alkyl group with coupling partners.
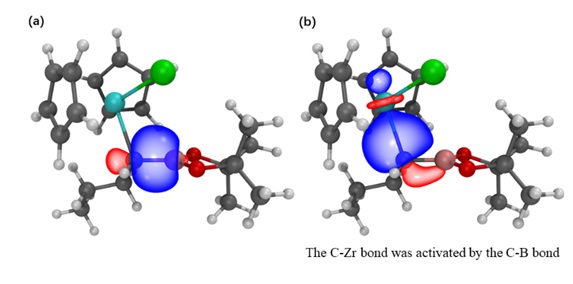
Based on extensive experimental studies,Dr.
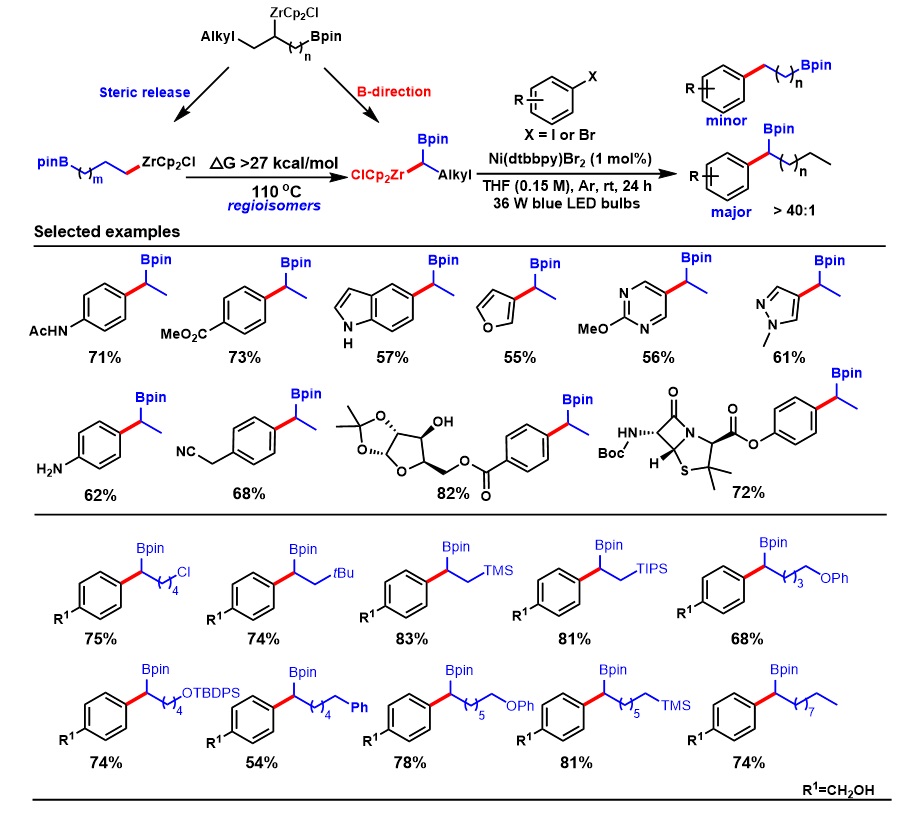
In summary,Dr.
Full text link:https://pubs.acs.org/doi/abs/10.1021/jacs.0c03821
Lab Link:http://qigroup.nibs.ac.cn/